Why will the crucible gain mass when heated.
If you’re searching for why will the crucible gain mass when heated pictures information linked to the why will the crucible gain mass when heated topic, you have come to the ideal blog. Our site always gives you suggestions for seeing the highest quality video and image content, please kindly hunt and find more informative video articles and images that fit your interests.
Why Will The Crucible Gain Mass When Heated. So all the Mg stays in the crucible but some oxygen forming MgO and a tiny bit of nitrogen. The iron wool has a much larger. If a crucible is not at the temperature of the balance compartment it will likely show a weight different from that it would show were it at the same temperature. Tiffanit8ay6ahy tiffanit8ay6ahy 01202017 Physics High School 5 pts.
 The Change In Mass When Magnesium Burns Experiment Rsc Education From edu.rsc.org
The Change In Mass When Magnesium Burns Experiment Rsc Education From edu.rsc.org
In the initial stage the gas temperature is in advance of the samplecrucible temperature. However this amount of air is not enough to ensure complete combustion of the Mg so the crucible lid must be intermittently raised but only ve. The balance has to be precise. Formation of magnesium oxide. If a question starts with the command word state give name or write down it needs a short answer only. If it is hotter the weight will be slightly less if colder slightly more owing to the production of convection currents which affect the apparent mass.
The iron wool has a much larger.
However this amount of air is not enough to ensure complete combustion of the Mg so the crucible lid must be intermittently raised but only ve. The buoyancy of the gas itself decreases and therefore the samplecrucible appears to gain weight. Porcelain crucibles are hygroscopic I. Get the answers you need now. The iron wool has a much larger. When the Mg is initially heated it begins to react with oxygen from the air present in the crucible.
 Source: pinterest.com
Source: pinterest.com
When the Mg is initially heated it begins to react with oxygen from the air present in the crucible. In the initial stage the gas temperature is in advance of the samplecrucible temperature. Please enable Javascript and refresh the page to continue. However the impurities are burned off in the experiment. Why will the crucible and its contents gain mass when heated.
 Source: pinterest.com
Source: pinterest.com
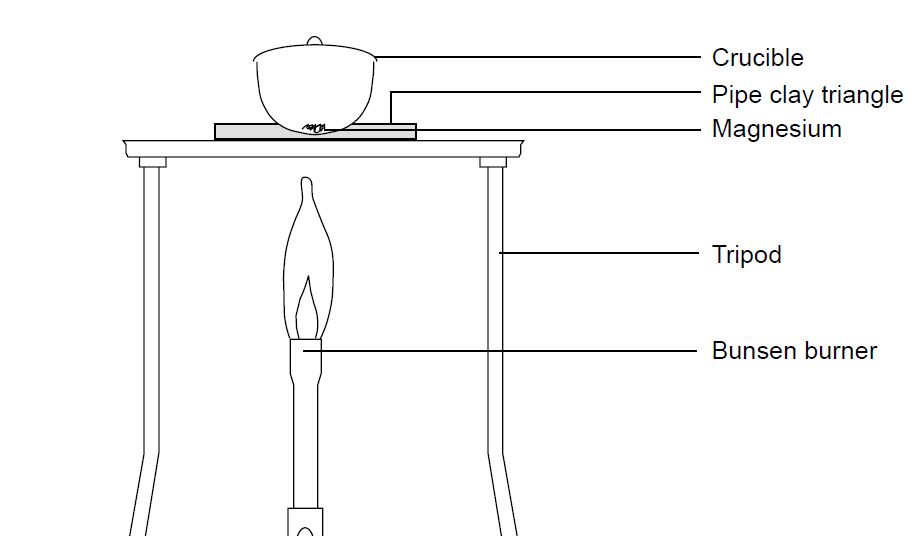
The magnesium reacts with oxygen to produce the oxide. Students see there is an increase in mass and can use the results to find the formula of magnesium oxide. What is the purpose of adding water during the experimental procedure. For this reason the porcelain crucible and lid is also pre-fired pre-heating to high temperature to constant mass before the pre-weighing. - The total mass will increase after heating process because magnesium will react with oxygen and form a new product called oxide.
 Source: mdpi.com
Source: mdpi.com
They are major sources of energy. Why will the crucible and its contents gain mass when heated. One reason is the greater surface area. If it is hotter the weight will be slightly less if colder slightly more owing to the production of convection currents which affect the apparent mass. However this amount of air is not enough to ensure complete combustion of the Mg so the crucible lid must be intermittently raised but only ve.
 Source: pinterest.com
Source: pinterest.com
Why will the crucible and its contents gain mass when heated. Get the answers you need now. The explanation for this goes beyond what is required at Key Stage 3 but it is summarised below. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. One of the most common methods of EA is combustion analysis where stance of interest is reacted with Oxygen.
 Source: mdpi.com
Source: mdpi.com
There are more atoms so the mass has increased. They are major sources of energy. The crucible is not fired as the procedure suggests but had retained some impurities from previous use or it could be oily smudges from fingers. Why will the crucible and its contents gain mass when heated. State and explain two reasons why hydrogen is very attractive fuel compared to fossils.
 Source: mdpi.com
Source: mdpi.com
This determines the mass of the completely dry crucible and lid. Questions with 1 2 3 or 4 marks usually start with command words. Because it contains a compound that forms a stable and solid oxide. The explanation for this goes beyond what is required at Key Stage 3 but it is summarised below. No the crucible will not gain mass because when you heat a substance it does not change during physical change.
 Source: pinterest.com
Source: pinterest.com
So then it reacts it gains x amount of oxygen atoms from the atmosphere. The iron wool has a much larger. The motion of the hot air upwards forces your crucible upwards but in small amounts. So then it reacts it gains x amount of oxygen atoms from the atmosphere. Formation of magnesium oxide.
 Source: pinterest.com
Source: pinterest.com
2 Empirical Formula Introduction tool of analytical chemistry is the ability to conduct Elemental Analysis EA to determine the percentat element present within a sample. If the crucible lid falls and breaks during heating do you have to start the experiment over again. Students may want to know why iron wool combusts but bigger bits of iron such as iron nails will not. 2 Empirical Formula Introduction tool of analytical chemistry is the ability to conduct Elemental Analysis EA to determine the percentat element present within a sample. No the crucible will not gain mass because when you heat a substance it does not change during physical change.
 Source: pinterest.com
Source: pinterest.com
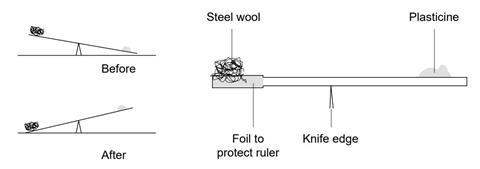
Heating in a crucible This method could be used for measuring mass loss in various thermal decomposition reactions and also for mass gain when reacting magnesium in oxygen. The explanation for this goes beyond what is required at Key Stage 3 but it is summarised below. The crucible is heated until the mass is constant to try to make sure that the reaction is finished. The crucible is not fired as the procedure suggests but had retained some impurities from previous use or it could be oily smudges from fingers. Students may want to know why iron wool combusts but bigger bits of iron such as iron nails will not.
 Source: prezi.com
Source: prezi.com
Students may want to know why iron wool combusts but bigger bits of iron such as iron nails will not. If a crucible is not at the temperature of the balance compartment it will likely show a weight different from that it would show were it at the same temperature. 2 Empirical Formula Introduction tool of analytical chemistry is the ability to conduct Elemental Analysis EA to determine the percentat element present within a sample. If the crucible lid falls and breaks during heating do you have to start the experiment over again. Why will the crucible and its contents gain mass when heated.
 Source: edu.rsc.org
Source: edu.rsc.org
The practical activity takes around 3045 minutes depending on. The crucible needs to be dry otherwise a wet crucible would give an inaccurate result. So all the Mg stays in the crucible but some oxygen forming MgO and a tiny bit of nitrogen. If a crucible is not at the temperature of the balance compartment it will likely show a weight different from that it would show were it at the same temperature. If the crucible lid falls and breaks during heating do you have to start the experiment over again.
 Source: pinterest.com
Source: pinterest.com
Tiffanit8ay6ahy tiffanit8ay6ahy 01202017 Physics High School 5 pts. Heating in a crucible This method could be used for measuring mass loss in various thermal decomposition reactions and also for mass gain when reacting magnesium in oxygen. The mass of the dirty crucible is recorded. If a question starts with the command word state give name or write down it needs a short answer only. This determines the mass of the completely dry crucible and lid.
 Source: pinterest.com
Source: pinterest.com
If a question starts with the command word state give name or write down it needs a short answer only. The crucible and crucible lid are both weighed together. The mass of the dirty crucible is recorded. If we put the hot crucible for measuring its mass convection currents will occur around the crucible where its in contact with the surrounding air. It would cause mass loss to be too large as the water would be lost when heating.
 Source: edu.rsc.org
Source: edu.rsc.org
Please enable Javascript and refresh the page to continue. They are major sources of energy. Porcelain crucibles are hygroscopic I. Why will the crucible and its contents gain mass when heated. The crucible needs to be dry otherwise a wet crucible would give an inaccurate result.
 Source: edu.rsc.org
Source: edu.rsc.org
They are major sources of energy. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. They absorb a bit of weighable moisture from the air. The crucible is not fired as the procedure suggests but had retained some impurities from previous use or it could be oily smudges from fingers. There are more atoms so the mass has increased.
 Source: pinterest.com
Source: pinterest.com
The crucible is not fired as the procedure suggests but had retained some impurities from previous use or it could be oily smudges from fingers. 2 Empirical Formula Introduction tool of analytical chemistry is the ability to conduct Elemental Analysis EA to determine the percentat element present within a sample. Because it contains a compound that forms a stable and solid oxide. Students weigh magnesium and heat it in a crucible. Heating in a crucible This method could be used for measuring mass loss in various thermal decomposition reactions and also for mass gain when reacting magnesium in oxygen.
 Source: link.springer.com
Source: link.springer.com
State and explain two reasons why hydrogen is very attractive fuel compared to fossils. The mass of the dirty crucible is recorded. Why will the crucible and its contents gain mass when heated. The crucible and crucible lid are both weighed together. Formation of magnesium oxide.
 Source: pinterest.com
Source: pinterest.com
If it is hotter the weight will be slightly less if colder slightly more owing to the production of convection currents which affect the apparent mass. There are more atoms so the mass has increased. Favorite Answer The Mg reacts with the oxygen in the air and a tiny bit of Mg reacts with N2. The crucible is heated until the mass is constant to try to make sure that the reaction is finished. January 16 2017.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why will the crucible gain mass when heated by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.







